Highlights
•
GS-441524 inhibited replication of serotype II FIP virus (FIPV) in CRFK cell cultures at an EC50 of approximately 1 uM and no toxicity at 100 uM.•
GS-441524 inhibited wildtype FIPV replication in macrophage cultures from ascitic fluid of two cats with naturally occurring FIP.•
GS-441525 is triphosphorylated by CRFK cells in vitro and PBMC in vivo.•
Pharmacokinetic studies in laboratory cats demonstrated effective blood levels over 24 h after a single dose of 5 mg/kg SC or IV.•
Severe experimental effusive FIP was successfully treated with 2 or 5 mg/kg GS-441524 SC q24 h for two weeks.
Abstract
Feline infectious peritonitis (FIP) is a common and highly lethal coronavirus disease of domestic cats. Recent studies of diseases caused by several RNA viruses in people and other species indicate that antiviral therapy may be effective against FIP in cats. The small molecule nucleoside analog GS-441524 is a molecular precursor to a pharmacologically active nucleoside triphosphate molecule. These analogs act as an alternative substrate and RNA-chain terminator of viral RNA dependent RNA polymerase. We determined that GS-441524 was non-toxic in feline cells at concentrations as high as 100 uM and effectively inhibited FIPV replication in cultured CRFK cells and in naturally infected feline peritoneal macrophages at concentrations as low as 1 uM. We determined the pharmacokinetics of GS-441524 in cats in vivo and established a dosage that would sustain effective blood levels for 24 h. In an experimental FIPV infection of cats, GS-441524 treatment caused a rapid reversal of disease signs and return to normality with as little as two weeks of treatment in 10/10 cats and with no apparent toxicity.
Keywords
Feline infectious peritonitis (FIP)FIP virus (FIPV)Nucleoside analogGS-441524Cell cultureEC50Tri-phosphorylationPharmacokineticsExperimental infectionLaboratory cats
1. Introduction
Feline infectious peritonitis (FIP) is a well-documented infectious disease of cats, especially kittens, adolescents and young adults from shelters, kitten foster/rescues and catteries (Pedersen, 2014b). The causative FIP virus (FIPV) is a positive single stranded RNA virus belonging to the family Coronaviridae, species Alphacoronavirus 1, subspecies feline coronavirus, biotype FIP virus (FIPV) (Tekes and Thiel, 2016).
FIPV infection has a complex immunopathogenesis, a feature shared by other coronavirus infections (de Wilde et al., 2017), and has become one of the most researched infectious diseases of cats since its discovery over a half century ago (Kipar and Meli, 2014; Pedersen, 2014a, 2014b; Tekes and Thiel, 2016). Despite these advances, modern treatment options for FIP remain palliative and vaccines have proven either unsafe or ineffective (Pedersen, 2014b). The emergence of SARS-associated coronavirus has provided an impetus for an investment in antiviral discovery focused on coronaviruses (De Clercq, 2004). A 3C-like protease inhibitor (GC376) was first demonstrated to be highly effective against experimental FIPV infections (Kim et al., 2016). A subsequent study on cats with naturally acquired FIP showed them to be much more difficult to treat (Pedersen et al., 2017). Nevertheless, this was the first study demonstrating the potential for an antiviral compound to treat FIP in nature and 6 of 20 cats have remained disease free for well over one year after 12 weeks of treatment (Pedersen et al., 2017).
GS-441524, a 1′-cyano-substituted adenine C-nucleoside ribose analogue (Fig. 1A ), is a small molecule that exhibits potent antiviral activity against a number of RNA viruses, including the zoonotic severe acute respiratory syndrome (SARS) coronavirus (Cho et al., 2012). A phosphoramidate prodrug of GS-441524 (GS-5734) has been previously shown to inhibit the replication of several taxonomically diverse RNA viruses such as Middle East respiratory syndrome virus, Ebola virus, Lassa fever virus, Junin virus and respiratory syncytial virus, while having low cytotoxicity in a wide-range of cell lines (Sheahan et al., 2017). GS-5734 has also been shown to protect rhesus monkeys from experimental Ebola virus infection (Warren et al., 2016).
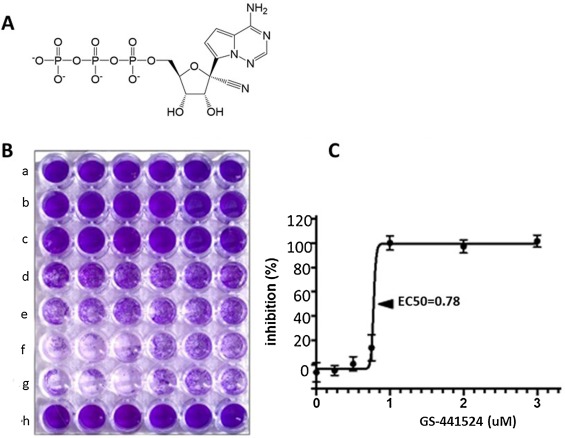
GS-441524 requires intracellular phosphorylation via cellular kinases to a nucleoside monophosphate and subsequently to the active triphosphate metabolite (NTP) (Cho et al., 2012; Sheahan et al., 2017; Warren et al., 2016) (Fig. 1A). The active NTP analog functions as a competitor of the natural nucleoside triphosphates in viral RNA synthesis. The active form of GS-441524 has been shown to inhibit RSV RNA-dependent RNA polymerase mediated transcription by incorporating into the nascent viral transcript and causing premature termination (Sheahan et al., 2017). We hypothesized that GS-441524 would be activated in feline cells, attenuate FIPV replication, have low cytotoxicity in feline cells in vitro and effectively treat cats with experimentally induced FIP.
2. Materials and methods
2.1. Experimental animals
Specific pathogen free (SPF) cats were purposefully bred in the Feline Research Laboratory (FRL) breeding colony of the Feline Nutrition Center, UC Davis. Twelve adolescent cats were used for experimental infections and six young adult cats for pharmacokinetic studies. Cats on experiment were housed in open rooms in facilities of the FRL and cared for by the FRL staff of animal caretakers. Experiments involving the use of laboratory bred cats were conducted under UC Davis IACUC protocol #19936. The UC Davis Policy on the Care and Use of Animals in Teaching and Research requires that University practices for the procurement, housing, and care and use of animals must conform to: 1) the ILAR Guide for the Care and Use of Laboratory Animals; 2) the Guide for the Care and Use of Agricultural Animals in Research and Teaching; 3) all regulations of the United States Department of Agriculture (USDA) issued by the USDA implementing the Animal Welfare Act (AWA) and its amendments (9 CFR, Chapter 3); and 4) the Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. In addition, University policy requires that all facilities in which animals are housed, and the programs associated with those facilities, must be accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), International.
2.2. FIPV-79-1146 inoculum for in vitro CRFK infection studies
Crandell-Rees feline kidney (CRFK) cells were propagated in 250 ml flasks with 25 ml DMEM/10%FBS and infected at 70% confluency with 1 ml cell-culture supernatant containing 5 × 105 tissue culture infectious doses-50% (TCID50) per ml of serotype II FIPV WSU-79-1146 (GenBank DQ010921) and incubated for 48 h. Flasks were frozen at −70 °C for 8 min and the thawed mixture of cells and original culture fluid centrifuged to remove cellular and subcellular debris and supernatant stored in liquid nitrogen. The level of infectious virus in an aliquot of the culture supernatant was then determined. CRFK cells were grown as described above in 24 well plates and infected with one ml/well of a serial 10-fold dilution of the frozen supernatant. Plates were then incubated for 48 h, stained with crystal violet (Fig. 1B), and each well was scored for CPE. A TCID50 was calculated from six replicates by the Reed-Muench method.
2.3. Quantitation of FIPV replication by qRT-PCR
Viral RNA was isolated from both the culture supernatant (QIAamp Viral RNA Mini Kit (Qiagen) and pelleted cells (RNAeasy, Qiagen) according to the manufacturer’s instructions. DNase treatment of isolated RNA was accomplished with TURBO DNase (Ambion). RNA was reverse transcribed into cDNA with the Origene First-Strand cDNA Synthesis System for qRT-PCR (Origene). A control reaction excluding reverse transcriptase was included for each sample. FIPV RNA copy numbers were measured by qRT-PCR as previously described (Murphy et al., 2012). PCR primers were based on a consensus sequence of the feline coronavirus 7b gene. The sequence for the forward primer was 5′-GGA AGT TTA GAT TTG ATT TGG CAA TGC TAG and the sequence for the reverse primer 5′-AAC AAT CAC TAG ATC CAG ACG TTA GCT. A control reaction excluding cDNA (water template) was included for each assay. Real-time PCR for the GAPDH housekeeping gene (GAPDH) was performed in parallel and results were normalized per 106 copies of GAPDH (13). Quantification of FIPV RNA copy number was based on a standard curve generated from viral transcripts prepared by in vitro transcription of a plasmid (pCR2.1, Invitrogen) containing a 112 nucleotide-long amplicon.
2.4. GS-441524
GS-441524 was provided by Gilead Sciences, Inc.
2.5. Analysis of cells and body fluids for GS-441524 prodrug and triphosphate
Proteins in samples of plasma, aqueous humor, and cerebrospinal fluid (CSF) were precipitated by adding acetonitrile to a final concentration of 89% in the presence of an internal standard [20 nM of 5-(2-Aminopropyl) indole (5-IT)]. Samples were filtered to remove precipitated protein, dried completely under a stream of nitrogen, and reconstituted with 0.2% formic acid 1% acetonitrile for LC/MS-MS analysis for GS-441524 concentrations. Frozen samples of cultured cells and PBMCs used for triphosphate analysis were resuspended in 0.5 ml of 70% methanol containing an internal standard (500 nM 2-chloro-ATP) for at least 30 min at −80 °C. The resulting supernatants were dried in a centrifuging evaporator (Genevac, Stone Ridge, NY) and samples were reconstituted with an aqueous solution of 1 mM ammonium phosphate, pH 7. Analytes were separated using a 50 x 2 mm, 2.5 u Luna C18(2) HST column (Phenomenex, Torrance, CA) connected to a LC-20ADXR (Shimadzu, Columbia, MD) ternary pump system and HTS PAL autosampler (LEAP Technologies, Carrboro, NC). A multi-stage linear gradient from 10% to 50% acetonitrile in a mobile phase containing 3 mM ammonium formate (pH 5.0) with 10 mM dimethylhexylamine at a flow rate of 150 u L/min was used to separate analytes. Detection was performed on an API 5000 (Applied Biosystems, Foster City, CA) MS/MS operating in positive ion and multiple reaction monitoring modes. Analytes were quantified using a 7-point standard curve ranging in concentration from 0.274 to 200 pM that was prepared from extracts of untreated cells.
2.6. Cyotoxicity of GS-441524 for CRFK cells
Cytotoxicity of GS-441524 was assessed using a commercially available procedure (CellTox Green Cytotoxicity Assay, Promega). Cytotoxicity was quantified by the intensity of fluorescence as the dye selectively binds the DNA of apoptotic/necrotic cells. The fluorescence of CRFK cells in 96 well plates and treated with 100, 33.3, 11.1, 3.7 and 1.2 uM concentrations of GS-441524 was compared to untreated CRFK cells (negative control) and cells treated with a cell lysing reagent provided by the manufacturer (positive control).
2.7. GS-441525 inhibition of FIPV replication in CRFK cells and in naturally infected macrophage cultures
Inhibition of virus replication by GS-441524 in either CRFK cells or naturally infected peritoneal macrophages was quantitated by visual/colorimetric inhibition of CPE as well as suppression of viral RNA expression by qRT-pCR. In the visual method, CRFK cells were grown in 96 well tissue culture plates containing 250 ul DMEM/10%FBS and 1.6 × 102 cells/well. Cells were infected at 70% confluency with 2.5 × 104 TCID50 of FIPV-79-1146/well and gently rocked for 60 min. GS-441524 was then added at concentrations ranging from 0.05 to 100 uM. Unattached cells were removed by gentle washing after 48 h and attached cells fixed with methanol and stained with crystal violet. Cytopathic effect was measured both visually (Fig. 1B) and by using a plate scanner for intensity of crystal violet staining. Tissue culture wells infected with virus alone (positive control) or uninfected wells (negative control) were run in parallel. CRFK cells for inhibition of viral RNA assays were cultured in six well plates and infected after 24 h with FIPV 79-1146 as described above, treated with 50, 10, 1.0, 0.1 or 0 uM GS-441524, and then cultured for a 20 h. Adherent cells were then gently detached with a cell scraper with the existing medium and culture supernatant freeze-thawed and tested by qRT-PcR. All assays were performed in six well replicates and the concentration of GS-441524 that inhibited infection by 50% (EC50) was determined by nonlinear regression analysis (PRISM 7, GraphPad).
The ability of GS-441524 to inhibit replication of wild type strains of FIPV in their natural host cell was determined in an ex vivo manner using qRT-PCR. Ten ml of ascites fluid was obtained by abdominocentesis from two client owned cats with naturally acquired effusive FIP and enrolled in a separate study (Pedersen et al., 2017). The fluid was diluted 1:1 with DMEM/10% FBS and aliquoted into two T25 tissue culture flasks at a volume of 10 ml/flask. The cultures were maintained for 24 h, which allowed peritoneal-type macrophages to attach to the flask and neutrophils and lymphocytes to remain free in the medium. The medium with detached cells was replaced, and cultures maintained for an additional 20 or 72 h. Adherent cells were gently removed with a cell scraper and medium and cells were freeze-thawed twice aliquots frozen at −70 °C.
2.8. Pharmacokinetic studies of GS-441524 in cats
A pharmacokinetic (PK) study was performed in laboratory cats to determine the metabolism and acute animal toxicity of GS-441524. GS-441524 was dissolved at a concentration of 12.5 mg/ml in 5% ethanol, 30% propylene glycol, 45% PEG 400, 20% water, and adjusted to pH 1.9 with concentrated HCl. Six laboratory cats were randomly divided into group A (n = 3; IV administration) or B (n = 3; SC administration). At time point zero, Group A cats were administered 5 mg compound/kg body weight intravenously while Group B cats received 5 mg compound/kg subcutaneously. Cats were then monitored for signs of acute toxicity (elevated pulse, respiratory distress, cyanosis, diarrhea, anorexia, drooling, vomiting, ataxia, weight loss, and changes in rectal temperature) daily for five days. Serial 0.5 ml whole blood samples in EDTA were obtained by lateral saphenous, superficial brachial or jugular venipuncture from each cat at 0.25, 0.5, 1, 2, 4, 6, 8, 12 and 24 h. After collection, blood samples were immediately placed on ice and then centrifuged at 5000 rpm for 5 min. The isolated plasma was pipetted into a 1.5 ml microcentrifuge tube and frozen at −70 °C for further analysis of free GS-441524. The buffy coat fraction from each blood collection was suspended in 1.5 ml phosphate free Tris-buffered saline (TBS, 50 mM Tris-Cl, pH 7.5, 150 mM NaCl) and PBMC isolated by Ficoll Hypaque density gradient centrifugation. The plasma and PBMC fractions were snap frozen in liquid nitrogen and shipped on dry ice to Gilead Sciences, Inc. (Foster City, CA) for further analyses. (plasma drug concentration and intracellular phosphorylation analyses).
2.9. Efficacy of GS-441524 against experimental FIP
Twelve six to nine-month-old SPF cats were experimentally infected by the intraperitoneal route with the cat-passaged serotype I FIPV-m3c-2 strain. The origin, cat-passage history, preparation of infectious inoculum, method of challenge-exposure, and monitoring for disease signs and response to treatment using this isolate have been previously reported (Kim et al., 2016; Pedersen et al., 2015). Non-treated control infection cats were not included, based on humane reasons and historical data on the outcome of identical challenge-exposures (Pedersen et al., 2015). Fever, absolute lymphopenia, inappetence, jaundice, and abdominal effusion appear 2–3 weeks after infection in around 80% of cats and rapidly increase in severity over the next one to two weeks and would prove fatal if no interventions were taken (Kim et al., 2016; Pedersen et al., 2015). Cats were treated once a day with either 5 mg/kg (Group A, n = 5) or 2 mg/kg (Group B, n = 5) GS-441524 for two weeks once their disease course became apparent, usually within three days. Some of the cats were treated with fluid replacement (50 m l lactated Ringer’s solution SC q24 h) and meloxicam (0.2 mg/kg PO q24 h) in this intervening period to prevent undue suffering. Ancillary treatment was stopped once GS-441524 treatment was instigated. Cats with recurrent disease signs, usually within four weeks after initial treatment, were treated with the same regimen for two additional weeks. All the cats were then observed for a minimum of 8 months for any recurrence of FIP.
2.10. Statistics
Data are presented as the mean of three or more values with the standard deviations displayed as error bars. An analysis of variance was performed (ANOVA) on each data set. Where global differences were identified, the Tukey-Kramer multiple comparisons test was utilized for pair-wise comparisons of the mean responses between treatment groups. A nonlinear regression analysis was performed for dose response data. Statistics were performed with PRISM 7 (GraphPad Software Inc., La Jolla, CA). A probability value ≤ 0.05 was deemed statistically significant.
3. Results
3.1. GS-441524 was not cytotoxic to CRFK cells at concentrations as high as 100 uM
Determining the toxicity of GS-441524 to CRFK cells was a prerequisite to all other studies. To make this determination, CRFK cells were treated with 100, 33.3, 11.1, 3.7 or 1.2 uM GS-441524 for 24 h. The cells appeared and grew normally at all concentrations of GS-441524 and failed to uptake the fluorescent dye CellTox Green at 24 h (data not shown). The cytotoxic concentration-50% (CC50) was therefore >100 uM. No cytotoxicity (CPE) was observed with CRFK cells exposed to 10 uM of GS-441524 for a longer period of 72 h, either visually or by quantitation of crystal violet staining.
3.2. GS-441524 inhibits cytopathic effect in CRFK cells as well as viral RNA expression in CRFK cells and naturally-infected peritoneal macrophages
It was crucial to show that GS-441524 inhibited FIPV replication in cultured cells at a very low concentration. This was done initially by infecting CRFK cell monolayers with serotype II FIPV-79-1146 and then treating with GS-441524 at concentrations ranging from none to 3.0 uM one hour later. The CRFK cells were protected from virus-induced CPE in a dose-dependent manner when tested 72 h later by crystal violet staining (Fig. 1B). This experiment was repeated three times with similar results and the effective concentration-50% (EC50) of GS-441524 was calculated to be 0.78 uM (Fig. 1C). Inhibition of FIPV replication by GS-441524 was also measured by qRT-RCR in CRFK cells infected with FIPV-79-1146 and exposed one hour later to 50, 10, 1.0, 0.1 and 0 uM GS-441524 for 20 h (Fig. 2A). Complete inhibition of viral RNA expression was seen at 50 and 10 uM, partial inhibition at 1.0 uM, and no inhibition at lower concentrations (p < 0.0001) (Fig. 2A).
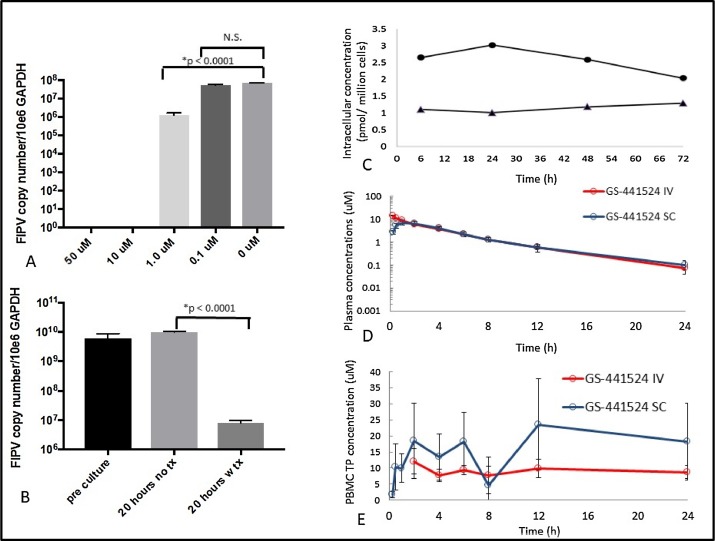
The serotype 2 FIPV-79-1146 strain has been adapted in vitro to grow on a few fibroblastic type cell lines. In comparison, Most FIPV isolates from nature are serotype 1 and have a specific tropism for peritoneal-type macrophages. As such, inhibition of FIPV-79-1146 replication in CRFK cells may not parallel the natural condition. To discount this possibility, ex vivo infected peritoneal macrophage cultures were established from ascites of two unrelated cats with naturally-acquired effusive FIP. Ex vivo cultures from the first cat were treated with 10 uM GS-441524 for 20 h, which resulted in a significant reduction of cell-associated FIPV RNA transcripts by approximately 1000-fold relative to untreated cells (Fig. 2B). This experiment was repeated with ascites cells isolated from a second cat with naturally-acquired FIP, but with the incubation extended to 72 h. These cultures also demonstrated significant (p < .0012) reduction in cell-associated FIPV RNA relative to untreated cells (data not shown).
3.3. Effective intracellular levels of non-phosphorylated and phosphorylated GS-441524 were sustained for three days in CRFK cells exposed to 1 uM GS-441524
It was important to demonstrate that GS-441524 is internalized by feline cells, that it is phosphorylated within cells to its active form, and that pro- and activated-drug levels are sustained. The intracellular levels of non-phosphorylated GS-441524 in CRFK cells exposed to 1 uM of the prodrug over a three-day period remained steady at 2 to 2.5 pM/million cells (Fig. 2C). Pharmacologically active triphosphate in these cultures was also tested and found to hold steady at concentrations around 1 pM/million cells over the three-day period. The intracellular volume of a typical cultured mammalian cell averages 1000 fL (1 pL) (Bryan et al., 2014), which would make the intracellular triphosphate level in a single cell 1 uM, which is near the EC50 calculated from CRFK cultures (Fig. 1C).
3.4. Effective blood levels of GS-441524 were sustained over 24 h following a SC or IV dose of 5 mg/kg and high levels of triphosphate were achieved in PBMCs
It was essential to determine the metabolism of GS-441524 in cats prior to animal infections studies. This was done with a standard pharmacokinetic (PK) study, where two cats were administered 5 mg/kg of GS-441524 subcutaneously and two cats received the same dose intravenously and plasma and cell (PBMC) levels of pro- and activated-GS-441524 measured over a defined time. The overall plasma pharmacokinetic profiles were similar between the subcutaneous and intravenous groups (Fig. 2D), with area under the curve (AUC) over 24 h values of 38.9 and 43.8 uM*hL, respectively. The intracellular triphosphate concentration in PBMC appeared higher in cats administered GS-441524 by the SC route, but the differences were not significant due to sample variation and number (Fig. 2E). The concentration of triphosphate in PBMC was sustained at 8–20 times higher than the levels of the prodrug (Fig. 2E). This was also 8–20 times higher than the EC50 of 1uM calculated by two different methods from CRFK cultures (Figs. 1C and 2A).
3.5. Concentrations of GS-441524 in aqueous humor and CSF were one-fifth that of plasma
FIP is well known to involve both the eyes and CNS (Pedersen, 2014b) and it is therefore important to know how well GS-441524 penetrates the blood/brain and blood/eye barriers. Two young adult cats were given 10 mg/kg of GS-441524 SC and two cats were untreated to provide control fluids. Samples of plasma (venous blood), aqueous humor (anterior chamber centesis) and CSF (cisterna magnum centesis) were collected two hours later and prodrug levels compared with plasma. Levels of the parent GS-441524 were highest in blood (11 and 12.8 uM), lower in ocular aqueous humor (2.4 and 4.3 μM or 22–33% of plasma), and lowest in the CSF (0.8 and 2.7 uM or 7-21% of plasma). Combined levels of drug in aqueous humor and CSF averaged 21.4% of blood plasma.
3.6. Two weeks of GS-441524 treatment at a dosage of 2 or 5 mg/kg SC q 24 h rapidly reversed clinical signs and prevented FIP-associated mortality in experimentally infected cats
The results of prior cell culture and PK studies indicated that GS-441524 would be an effective treatment for FIP in cats and suggested an effective dosage regimen. This was tested in an experimental infection study where laboratory cats were inoculated intraperitoneally with a strain of FIPV that has been maintained as near to wild-type as possible and well characterized in past studies (Kim et al., 2016; Pedersen et al., 2015). Twelve adolescent laboratory cats were challenge-exposed to this FIPV and their response to GS-441524 treatment monitored when terminal disease signs became apparent. Ten of 12 of these cats, 16–113, 16-116, 16-119, 16-123 and 124, 16-127 to 131, demonstrated clinical signs consistent with FIP within 10-18 days, while two cats (16-115 and 16-118) remained healthy (Fig. 3). Clinical signs of FIP in the affected cats started with hyperthermia (>103 F) (Fig. 4) and lymphopenia (less than or equal to 1700 cells/ul blood) (Fig. 5), and then rapidly progressed to depression, anorexia, hyperbilirubinemia, and ascites. The severity of lymphopenia appeared to be proportional to the severity of overall disease signs and one cat (16–113) had a measured nadir of 143 lymphocytes/ul blood (Fig. 5). Rectal temperature and lymphocyte levels remained normal in the two asymptomatic cats.
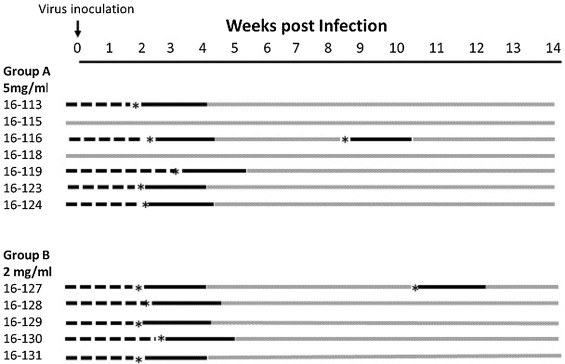
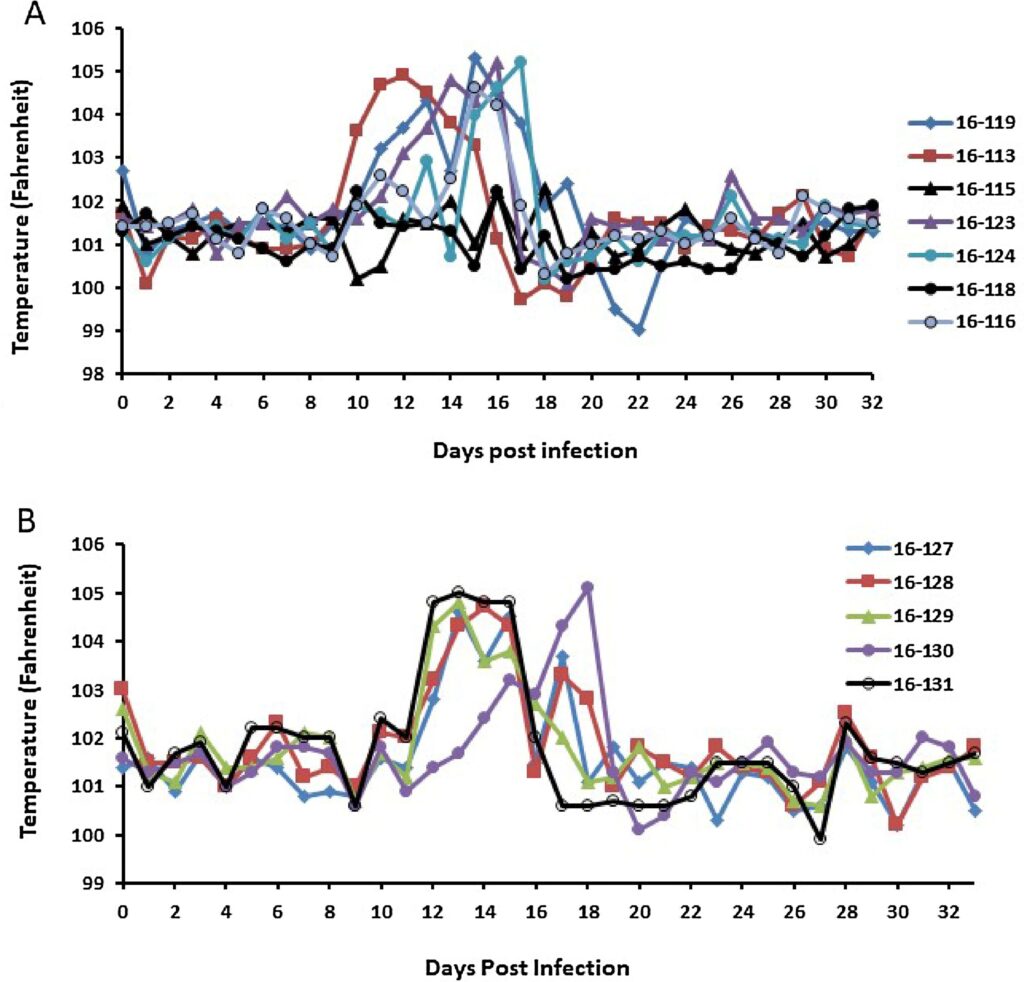
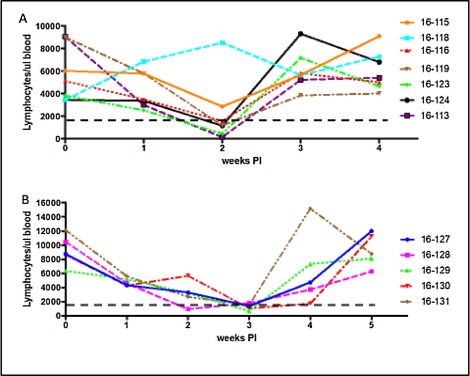
The 10 cats that developed disease signs were divided into two groups and treated with either 5 mg/kg (Group A; n = 5) or 2 mg/kg (Group B; n = 5) GS-441524 SC q24 h starting three days after unequivocal clinical evidence of FIP (days 12–19 post infection) (Fig. 3, Fig. 4, Fig. 5). The two cats that did not develop disease signs served as controls for normal blood lymphocyte counts and rectal temperature. All 10 treated cats had a rapid response to treatment and lymphocyte levels and rectal temperatures returned to pre-infection levels and levels of the two asymptomatic cats (Figs. 4,5). Two of the 10 treated cats, 16–116 (Group A) and 16–127 (Group B), had recurrences disease at four and six weeks post treatment (Fig. 3). These two cats were treated a second time for two weeks and their response was identical to that of primary treatment (data not shown). All ten of the once or twice treated cats have remained normal to date (more than eight months post infection). No significant signs of toxicity were noted during or after primary or secondary treatment. Injections caused a transient “stinging” reaction in some cats within 10 s of compound administration. Localized and transient pain was evidenced by unusual posturing, licking at the injection site and/or vocalizations that lasted for approximately 30–60 s after injection. Injection reactions were more pronounced in some animals relative to others and reactions were inconsistent from one injection to the next and decreased over time.
4. Discussion
The biologic activity of the prodrug GS-441524 against FIPV infection was studied in progressive stages. The initial step involved screening potential compounds and identifying ones with favorable EC50 and CC50 values. This was done in vitro using CRFK cells and a tissue culture adapted serotype II FIPV (FIPV-WSU-79-1146). CRFK infection experiments identified GS-441524, which had a low EC50 (0.78 uM) against FIPV-79-1146 and no cytotoxicity at the highest concentrations tested (100 uM). The next step involved an ex vivo experiment using naturally infected peritoneal macrophage cultures established from two field cases of effusive FIP. GS-441524 was equally effective in inhibiting wild-type serotype I FIPV replication in naturally infected peritoneal cells than it was in inhibiting FIPV-79-1146 in CRFK cells. Therefore, we anticipated that GS-441524 would also be effective in cat infection studies against the virulent non-tissue culture adapted serotype 1 FIPV-m3c-2 strain.
The second stage was to conduct a PK study to rule out acute toxicities in cats treated with GS-441524 and to determine an optimal dosage regimen based on blood and tissue distribution. No acute toxicities were observed in laboratory cats after a single SC or IV injection of GS-441524. Subcutaneous or intravenous dosage at 5 mg/kg GS-441524 resulted in similar plasma profiles and the intracellular triphosphate levels were approximately 8–20 uM in feline PBMCs over a 24 h period. The occurrence of neurological and ocular disease in cats FIP (Pedersen 2014b), and CNS complications in an earlier trial of a viral protease inhibitor (Pedersen et al., 2017), was impetus for a supplementary PK experiment testing the ability of GS-441524 to pass the blood/eye and blood/brain barriers. A single SC dose of GS-441524 at 10 mg/kg resulted in intraocular and CNS drug levels of the parent GS-441524 that were near or above the effective 1 uM level. Whether this amount of GS-441524 entering eyes and brain can cure disease in these sites must await field testing, as it is extremely difficult to recreate and study ocular and neurologic diseases in the laboratory. GC376, a 3 C-like protease inhibitor, crossed the blood-to-brain barrier at a CSF/plasma ratio comparative to GS-441524 (Kim et al., 2015), but it was not effective against pre-existing neurological disease or in preventing eventual CNS involvement in a proportion of treated cats (Pedersen et al., 2017).
In the final stage, 12 laboratory-bred cats were experimentally infected with a cat-to-cat passaged serotype 1 strain of FIPV and 10 of them developed severe disease signs. Five of these ill cats were treated with 2 mg/kg doses of GS-441524 and five with 5 mg/kg given subcutaneously every 24 h. A dosage of 5 mg/kg of the parent drug sustained blood levels over 24 h in the PK study, which was 8–20 times higher than an EC50 of the calculated from cell culture experiments. Therefore, it was reasonable to postulate that 2 mg/kg would also be effective and conserve drug and reduce potential toxicity. The 10 treated cats manifested a rapid reversal of disease signs starting within 24–48 h and comparable between dosages. After two weeks of treatment all 10 cats were well back to normal health and the treatment stopped. Although no uninfected/untreated control cats were used in the study, spontaneous recovery is extremely uncommon among either experimentally induced (Pedersen et al., 2014) or naturally-occurring FIP (Ritz et al., 2007; Fischer et al., 2011). The results of this cat infection study mirrored our previous experience with a 3C-like protease (Kim et al., 2016).
Two treated cats, one in each dosage group, experienced disease relapses at four and six weeks after treatment ended. A second two-week course of GS-441524 resulted in rapid resolution of disease signs. Relapses following protease inhibitor treatment (Pedersen et al., 2017) and experimental infection (de Groot-Mijnes et al., 2005) have been observed and are most likely due to incomplete clearance of the infection and/or a failure to mount a protective immune response during infection. The ten treated cats have remained clinically normal to date (eight months post infection). No systemic signs of drug toxicity were noted in any of the cats over two-week, and two-week plus a second two-week, treatment periods.
Studies with tissue cultured cells and laboratory cats strongly support further testing of GS-441524 in client-owned cats with various forms of naturally-acquired FIP. An earlier study with a novel 3 C-like protease inhibitor (GC376), which also underwent the same types of pre-testing (Pedersen et al., 2017), has provided a template for such a field trial. Field testing of GC376 demonstrated that naturally occurring FIPV infection is more difficult to treat than natural infection, may require a somewhat higher dose, involves many more weeks of treatment, and the cure rate is apt to be lower. Although 19 of the 20 field cats treated with GC376 regained outward health within two weeks of treatment, only 6 cats have remained disease free after more than 18 months (Pedersen et al., 2017).
5. Conclusion
The nucleoside analog GS-441524 completely inhibits FIPV replication in CRFK cells and naturally infected feline peritoneal macrophages in vitro concentrations ≥1 uM and with no detectable toxicity at 100 uM. A dosage regimen was determined from a pharmacokinetic study in laboratory cats and employed to successfully treat normally fatal experimentally-induced disease. Nucleoside analogs such as GS-441524 hold great promise in the treatment of naturally occurring FIP and may have application to studies of coronavirus infections of other species.
Funding statement
In vitro studies of Dr. Murphy and Dr. Pedersen involving the use of cell cultures to screen compounds for anti-coronavirus activity and lack of toxicity were funded by a grant from the Winn Feline Foundation. Funds for follow-up studies involving laboratory cats were provided by the Center for Companion Animal Health through gifts specified for FIP research by numerous individual donors, organizations (SOCK FIP, Davis, CA) and Foundations (Philip Raskin Fund, Kansas City, KS).
Declaration of interest
M. Perron, E. Murakami and Y. Park are employees of Gilead Sciences, Inc., Foster City, CA, USA and hold stock interests in the company.
Acknowledgements
We would like to acknowledge Hongwei Liu, Andre Poon and Andrew Cohn (UC Davis) for assistance with the experiments, data analysis and graphics, Lars Heumann, Kelly Eberle, Morin Frick and Sean Neville (Gilead) for help with compound synthesis, and D. Babusis (Gilead) for his help for PK and metabolism analyses. We are also grateful for Maria Moreno and her staff at UC Davis for the care of the cats and Kirsten Murphy for manuscript editing. GS-441524 and drug and drug-metabolite assays were kindly provided by Gilead Sciences, Inc. (Foster City, CA).